News & Perspectives
Filter
Add filters
Category
TCV News
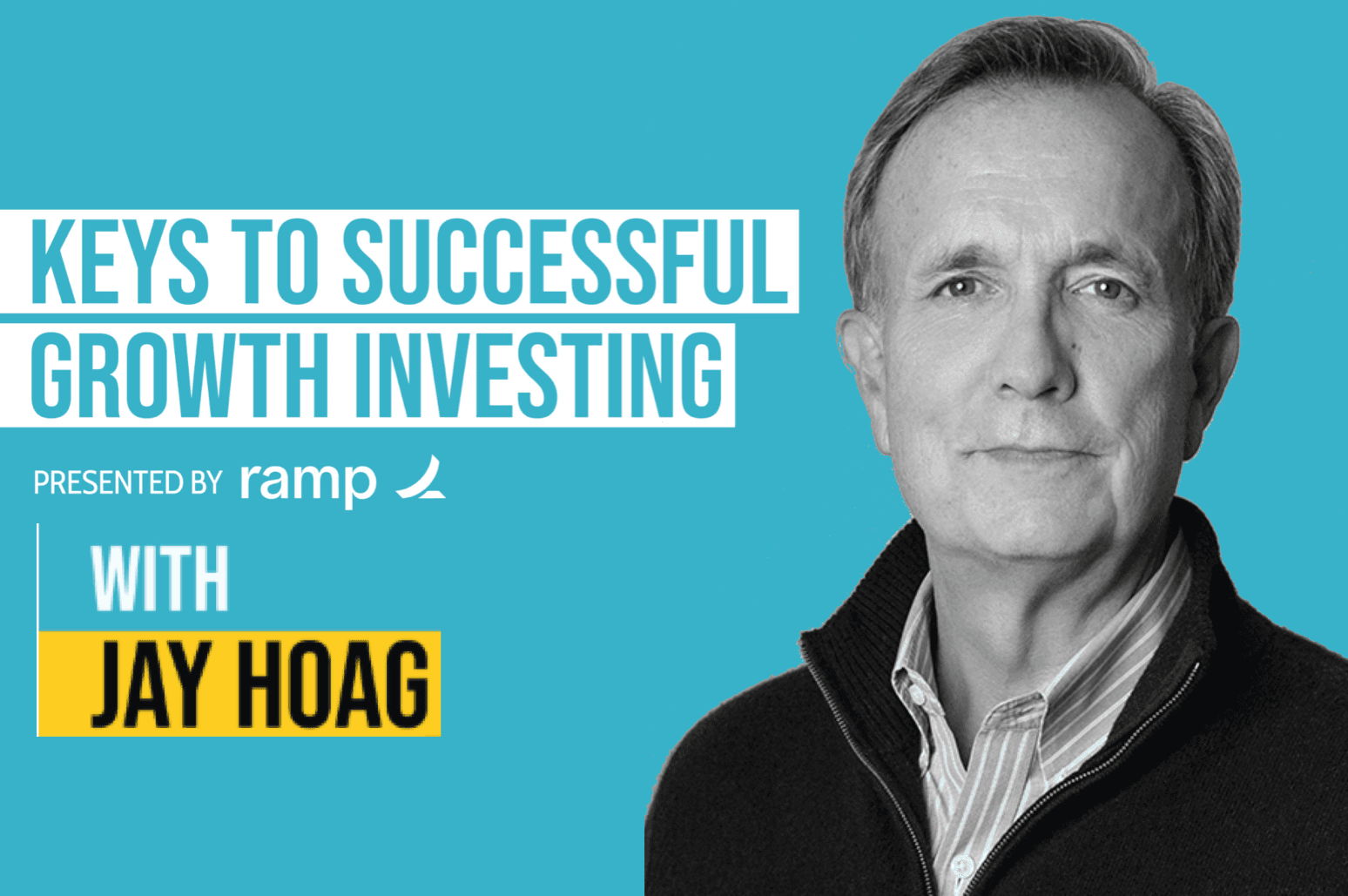
Jay Hoag on the Invest Like the Best Podcast with Patrick O'Shaughnessy

Portfolio News
Nasuni: Setting the Standard for the Hybrid Cloud Era
Portfolio News
Building the Modern Software Supply Chain: Our Investment in Cloudsmith
TCV News
Insights from Strategic Advisor Bob Burke: Our Investment in RELEX Solutions

Portfolio News
Adevinta: Pan-European Online Classifieds
TCV News
TCV’s Founding General Partner Jay Hoag and General Partner John Doran join Live From MarketSite with Nasdaq's Kristina Ayanian

Thought Leadership
Full Potential SaaS: An Update

Portfolio News
Muz Ashraf on TCV’s Investment in Employment Hero
Portfolio News